|
This topic was also presented as a Poster:
see the short abstract and the PDF (DOI 10.3247/SL4Nmr12.004).
Long abstract
The idea presented here falls somewhere between "totally crazy" and "how comes we have overlooked it". I have already hinted at it at last year's meeting of the Italian GIDRM [1] and this presentation is the first one to expand on it in more detail.
Consider a planar diamagnetic molecule which has an axial sense, such as that of chloraldehyde or, in general C(XYZ), where C is a central atom and XYZ are three different atoms/groups arranged around it (see Fig1):
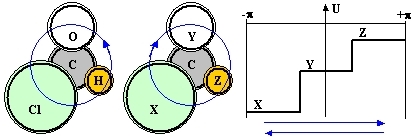
Figure 1: A bonding-shell electron shared by three different atoms XYZ is a bit like a particle constrained to a circular path and moving under the influence of a potential U (solid line) with no circular symmetry. In this case it evidently matters whether its wavefunction propagates clockwise or counter-clockwise (blue arrows) and the energies of the corresponding eigenstates are different.
Obviously, the axiality of the arrangement due to the conceptual difference between a clockwise and a counter-clockwise circumnavigation of the (XYZ) atoms can not lead to distinct molecular species (such as in chiral arrangements). But it does define an axial vector well oriented with respect to the common plane, a fact compatible with the existence of a preferential direction of motion and a persistent electron current loop. A bond electron partially shared by all three atoms can in fact circulate around the structure either in one sense (XYZ), or in the other sense (XZY) and, when XYZ are all different, these two types of loop orbits have distinct energies. In practice, such loop orbits will mix with the conventional electron orbitals and thus convey to them an axial character and contribute in part to the energy of the molecule. From the symmetry point of view, the axiality needs to be accounted for in any math model of the molecule, lest an important feature of the latter is a-priori missed.
As far as the author is aware, the present quantum theory of chemical bonding never considers any axial aspects. They are absent in models based on combinations of electron Slater-type orbitals, because the latter are inherited from two-particle models (electron and a nuclide), while axiality requires at least four bodies (electron and three nuclides). Nor are they accounted for in DFT [2] which considers only electron densities, but no persistent electron current loops. This overlooked factor might yet explain the scarce progress of quantum chemistry. For example, the errors in quantum predictions of chemical shifts are today only 2-3 times lower than 40 years ago, despite the huge progress in computing technology. This makes sense only if one admits that a cog is missing somewhere inside the quantum chemistry bluprints. Which might well be the need to complement electron charge density with an electron current density.
Another telltale indication is that we all know about electric dipoles of molecules and their fragments, both permanent and induced, and we are also aware of induced magnetic dipole moments in molecules, such as those responsible for diamagnetic susceptibility, or for the induced ring currents evidenced by NMR chemical shifts. However, permanent magnetic moments in diamagnetic molecules [3] have never been contemplated - without any apparent reason!
Why should the idea of persistent electron current loops in diamagnetic molecules and in their fragments be presented at this meeting? The answer is that if the insight is correct then some molecules, just like some elementary particles and some nuclides, should possess an intrinsic spin (an orbital one) and an associated permanent magnetic moment, thus giving rise to magnetic resonance phenomena similar to those we are used to (in particular a specific Larmor frequency). We are therefore talking about the possibility of extending radically the range of "particles" endowed with spin properties and gyromagnetic moments and giving birth to Molecular Magnetic Resonance (MMR).
However, before starting the search for such molecular spins, one should try and estimate the energy difference between axially distinct electronic states, their lifetimes, inter-conversion rates, Larmor frequencies, and MMR relaxation times. Some of this physics, based on grossly simplified quantum models, will be presented. Even so, the search itself may be anything but trivial because, with no firm advance knowledge of the Larmor frequencies, it will entail extremely broad-band hardware approach. Let this presentation be a call to arms to conquer this New World!
Please, cite this online document as:
Sykora S.,
Molecular Spins: a novel Frontier of Chemical Physics and Magnetic Resonance?,
Poster at 53rd ENC, Miami, April 15-20, 2012, DOI: 10.3247/SL4Nmr12.005.
References
[1] Stan Sykora, Gold Medal Talk at the 40th Annual Meeting of GIDRM, September 26-28, 2011, Parma, Italy.
[2] Wikipedia, Density functional theory; and references therein
[3] The well known permanent magnetism due to unpaired electron spins is not discussed here at all.
Discussions
Your comments are welcome and will appear here
|